H.-S. Jeong†, J. Kim†, K.-I. Jo, J. Kee, J.-H. Choi, J. Koo
Carbon Letters, Accepted (2020)
First-principle calculation: Insight for Material
First-principle calculation is the method which of calculating physical, chemical or electrical properties by using quantum mechanics based on only basic information of a material. In the field of battery research, first principles calculations are used, such as predicting the operating voltage at which ions are intercalated or visualizing the ion diffusion path. Through this method, it is possible to quickly derive a material having a desired characteristic, thereby reducing time and cost expenses.

▶ Calculated structures of end-member phases.
(J. Kim et al,, Chem. Mater. 2018, 30, 3683.)

▶ Na diffusion and related calculation in a-FePO4
(J. Kim et al., Energy Environ. Sci. 2015, 8, 540..)
Lithium-ion battery: Best energy storage system
Environmental issues such as the depletion of fossil fuels and the fine dust pollution problem have driven the demand for ecofriendly and sustainable energy storage systems such as rechargeable batteries. Currently, lithium-ion batteries (LIBs) are widely used as the main power source in applications ranging from portable electronics to grid-scale energy storage systems because of their high energy density and satisfactory cycle life. In addition, the recent shift from internal combustion engines to electric vehicles (EVs) has accelerated the demand for high energy-density LIBs.
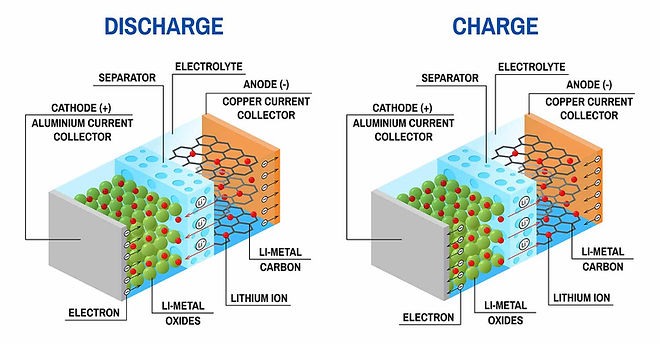
▶ Operation schematic of LIB
(https://www.malvernpanalytical.com/kr/industries/advanced-materials/batteries)
Alternatives to LIB for grid-scale energy storage system
: Low-cost Na & K Ion Batteries
Recently, sodium-ion batteries (SIBs) have been considered one of the promising alternatives to LIBs because of the virtually unlimited Na resources and their analogous reaction mechanism to LIBs based on the de/intercalation of mobile alkali ions in the structure.
And potassium-ion batteries (KIBs) also have attracted particular attention as promising alternatives to LIBs because of the abundance of global potassium resources and the lower standard redox potential of potassium compared with that of other metallic elements (Eº(Li/Li+ ): 3.04 V; Eº(K/K+ ): 2.93 V; Eº(Na/Na+ ): 2.71 V; vs. the standard hydrogen electrode (SHE)).
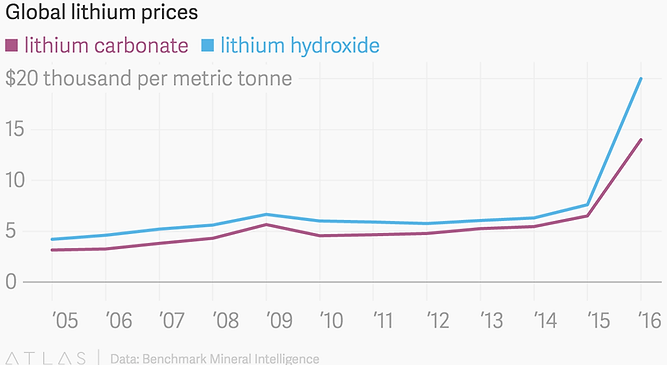
▶ Price of lithium resources
(https://www.infiniteenergy.com.au/lithium-price-increase-and-what-it-means-for-you/)
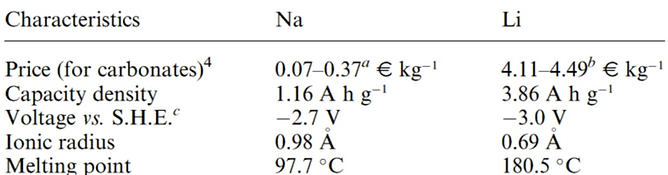
▶ Characteristic comparison between Na and Li
(https://www.infiniteenergy.com.au/lithium-price-increase-and-what-it-means-for-you/)